
According to the Bohr model, whenever a hydrogen atom changes from
one stationary state to another, energy is emitted or absorbed. When
that energy takes the form of electromagnetic radiation then it has a
frequency f (or as it is often called,
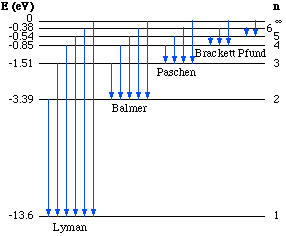
where Ef and Ei are the final and initial energies respectively. If Ei > Ef, then radiation occurs while if Ef > Ei, then absorption takes place. The energy level diagram of the hydrogen atom is shown to the left.
In the diagram the first six levels are shown as well as the zero
energy level (n = ![]() ).
Various transitions are shown as vertical blue arrows. If the
transition takes place from any n to n =1, it is referred to as a
Lyman line. Transitions to n = 2 are called Balmer lines and so on.
Four of the Balmer lines are the visible range.
).
Various transitions are shown as vertical blue arrows. If the
transition takes place from any n to n =1, it is referred to as a
Lyman line. Transitions to n = 2 are called Balmer lines and so on.
Four of the Balmer lines are the visible range.
© MultiMedia Physics 2000