 A
photon and an electron do not have the same wavelength when they have
the same energy. For example here is a calculation of wavelength for
a photon and an (nonrelativistic) electron with the same kinetic
energy:
A
photon and an electron do not have the same wavelength when they have
the same energy. For example here is a calculation of wavelength for
a photon and an (nonrelativistic) electron with the same kinetic
energy:
 A
photon and an electron do not have the same wavelength when they have
the same energy. For example here is a calculation of wavelength for
a photon and an (nonrelativistic) electron with the same kinetic
energy:
A
photon and an electron do not have the same wavelength when they have
the same energy. For example here is a calculation of wavelength for
a photon and an (nonrelativistic) electron with the same kinetic
energy:
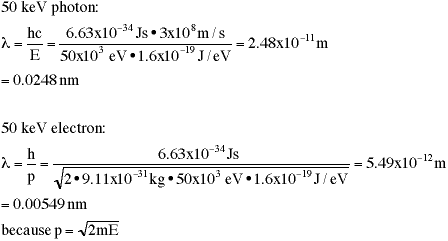
simplifying the above equations gives the following shortcuts:
l (nm) = 1240/E(eV) for photons
l (nm) = 1.228/E1/2(eV) for electrons
But they do have the same wavelength if they have the same momentum!
© MultiMedia Physics 2000