Kinetic Theory
In the kinetic theory of gases, the concept is of a large number
of randomly moving molecules, which bounce off each other and the
walls of the vessel containing the gas. The concepts of pressure
and temperature follow naturally from this
model. Pressure is due to the bouncing of the molecules off the
walls, and temperature is a measure of their average kinetic
energy.
 Molecules
are in continuous random motion.
Molecules
are in continuous random motion.- They are point particles in that their volume is much smaller
than the volume of the gas.
- No forces except during collisions.
- Collisions with each other and the walls are elastic.
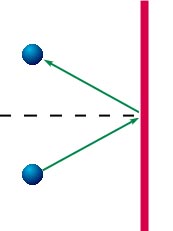
- The force on the wall is due to collisions, as the figure on
the right shows: molecules reverse their momentum component
perpendicular to the wall during the collision with the wall,
whereas the component along the wall remains the same. This
reversal of the momentum is an impulse,
and from momentum conservation,
the wall will receive the opposite impulse.
- The average force on the wall is then the sum of all impulses
received by the molecules from the wall during a given time
interval, divided by that time interval:
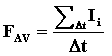
© MultiMedia
Physics, 1999
 Molecules
are in continuous random motion.
Molecules
are in continuous random motion.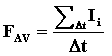
 Molecules
are in continuous random motion.
Molecules
are in continuous random motion.